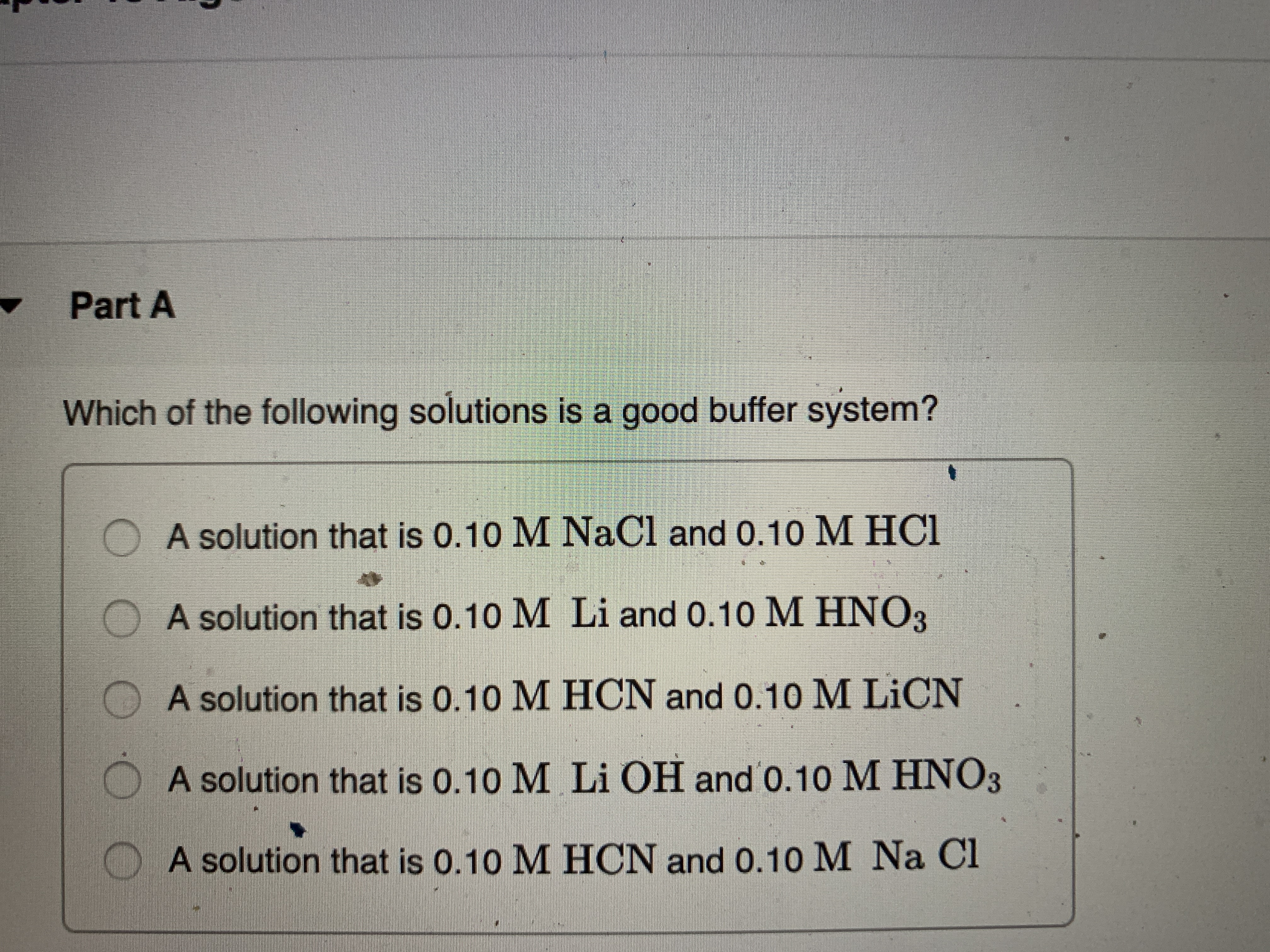
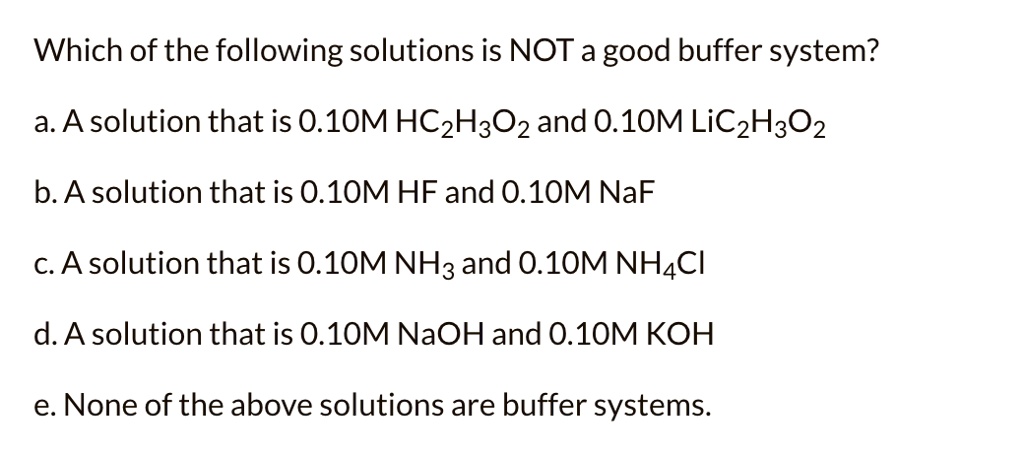
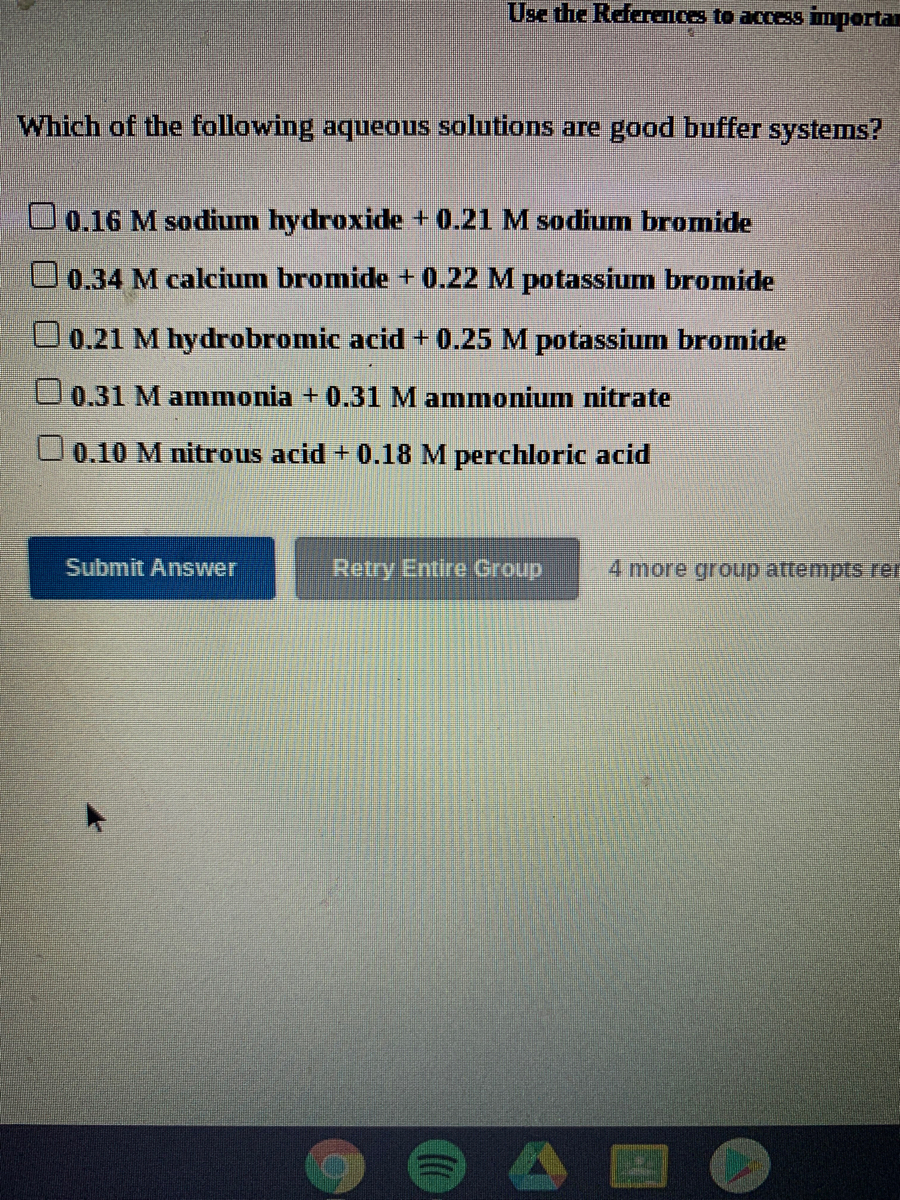
Which Of The Following Solutions Is A Good Buffer System?
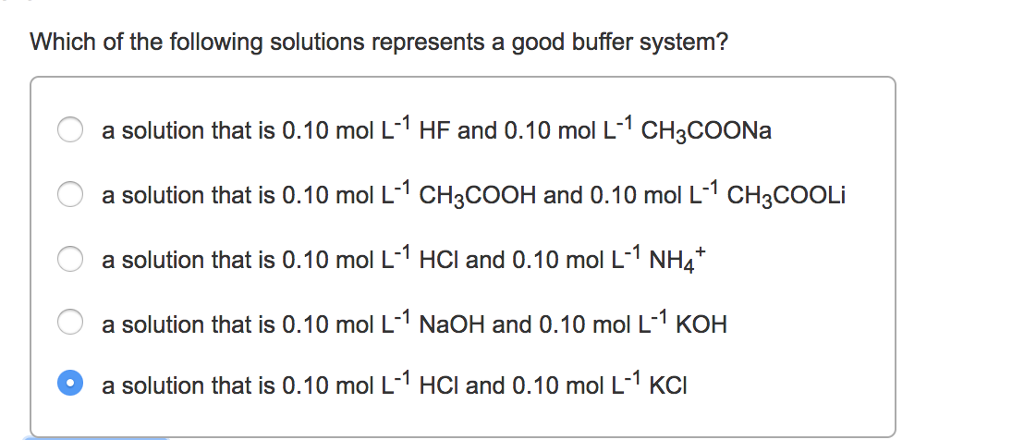
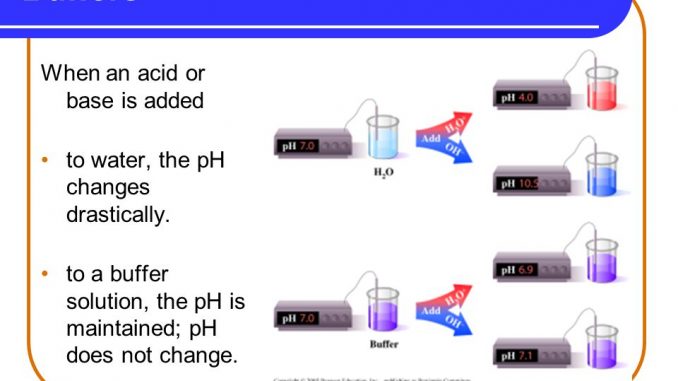
Which of the following solutions is a good buffer system?. Which of the following solutions is a good buffer system. It is not a buffer because HCl is a strong acid. 020 M sodium hydroxide 021 M sodium chloride b.
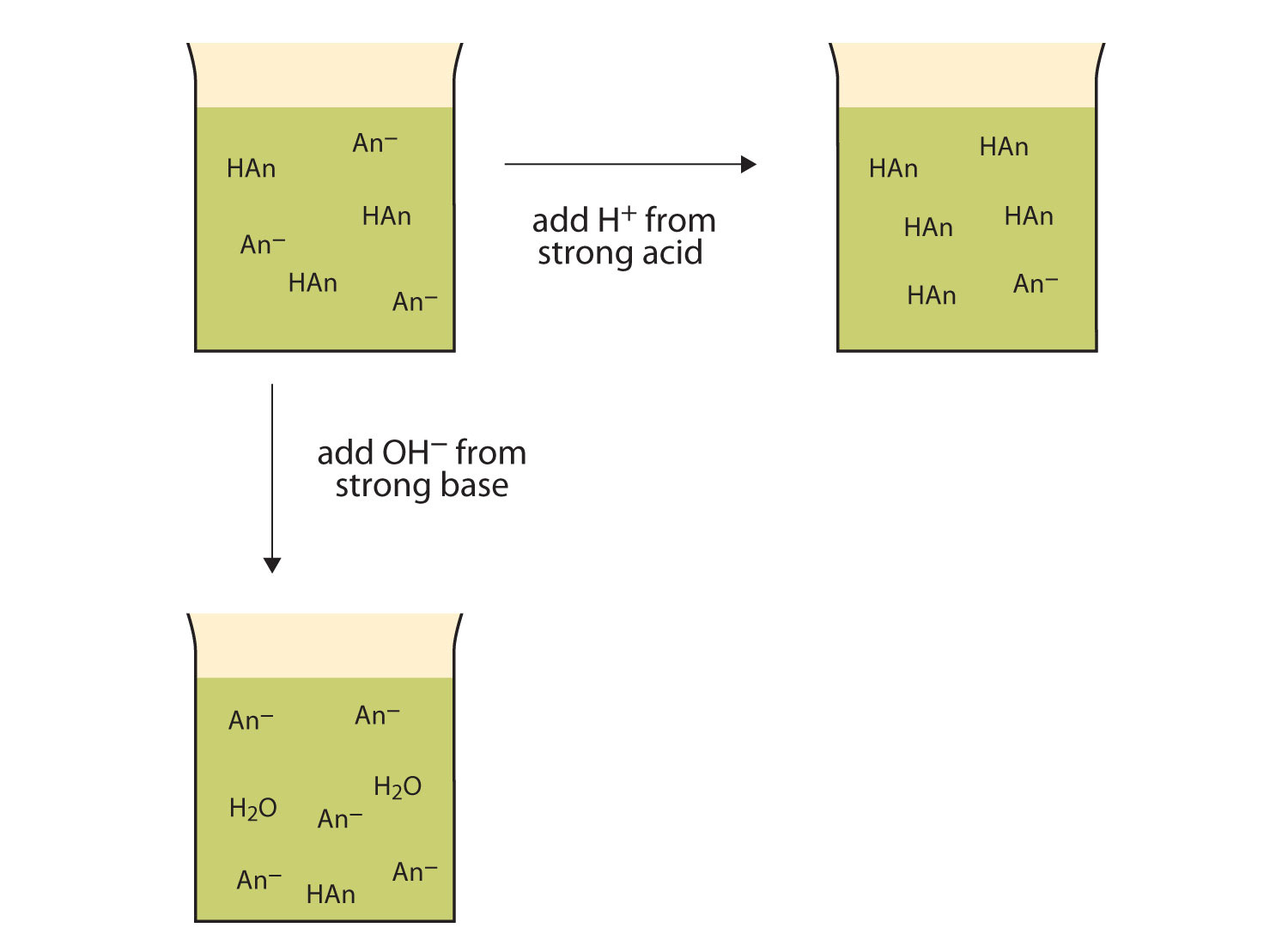
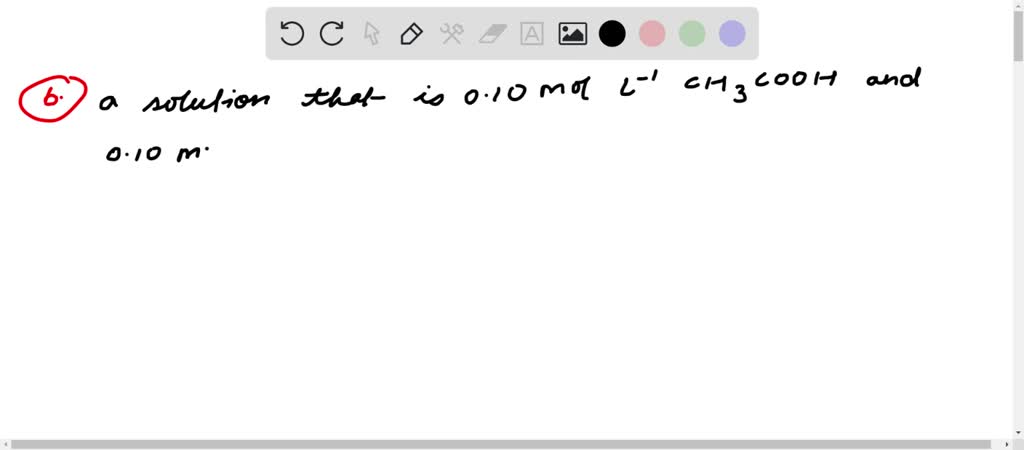
J024 M hydrochloric acid 020 M potassium chloride 019 M acetic acid 016 M sodium acetate 035 M calcium iodide 024 M barium iodide O 020 M barium hydroxide 029 M barium chloride O. A buffer solution contains a weak acid or base and the conjugate base or acid A solution that is 010 M HC2H3O2 and 010 M LiC2H3O2The solution has the same amount of moles in the weak acid and conjugate base. A a solution that is 010 M NaOH and 010 M HNO 3 NaOH strong base HNO 3 strong acid strong acid strong base not a buffer not a good buffer system.
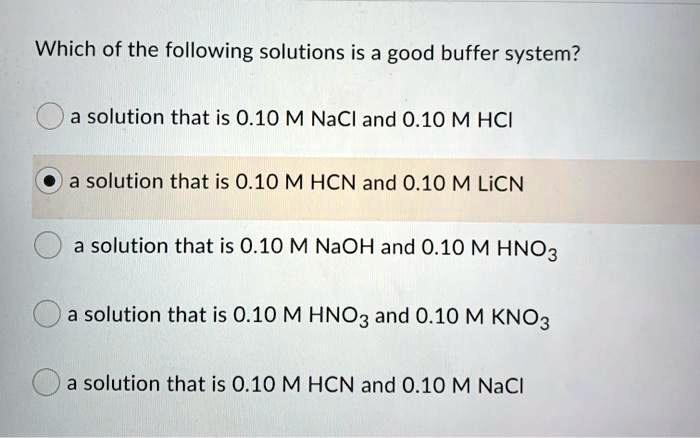
Since all of the given solutions have the same 010 M concentrations lets take a look at what are they composed of. 038 M calcium bromide 030 M barium bromide. A solution that is 010 m hcn and 010 m nacl a solution that is 010 m hcn and 010 m licn a solution that is 010 m hno3 and 010 m kno3 a solution that is 010 m naoh and 010 m hno3 a solution that is 010 m nacl and 010 m hc.
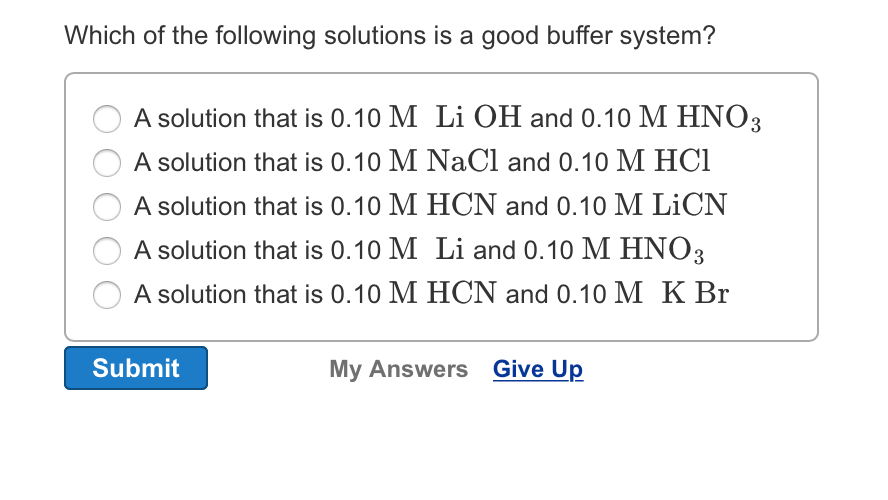
B a solution that is 010 M NaCl and 010 M HCl. B A solution that is 010 M HCN and 010 M LiCN. 028 M hydroiodic acid 018 M sodium iodide.
A solution that is 010 M NaOH and 010 M HNO3 D. A good buffer system is given by. A solution that is 010 M HCNl and 010 M LiCN C.
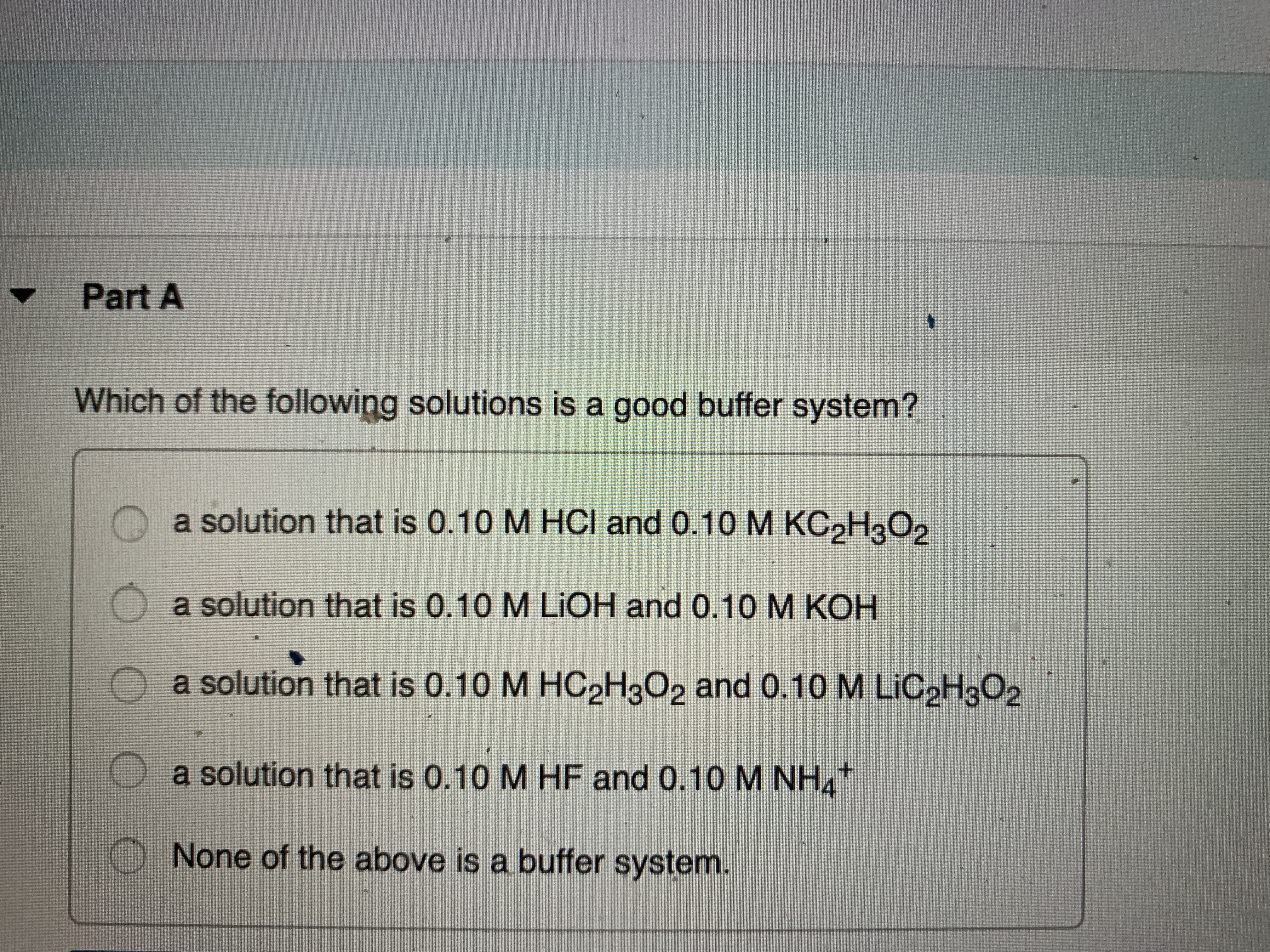
C a solution that is 010 M HCl and 010 M. A solution that is 010 M NH3 and 010 M NH4Cl B. 010M HC 2 H 3 O 2 and 010M LiC 2 H 3 O 2.
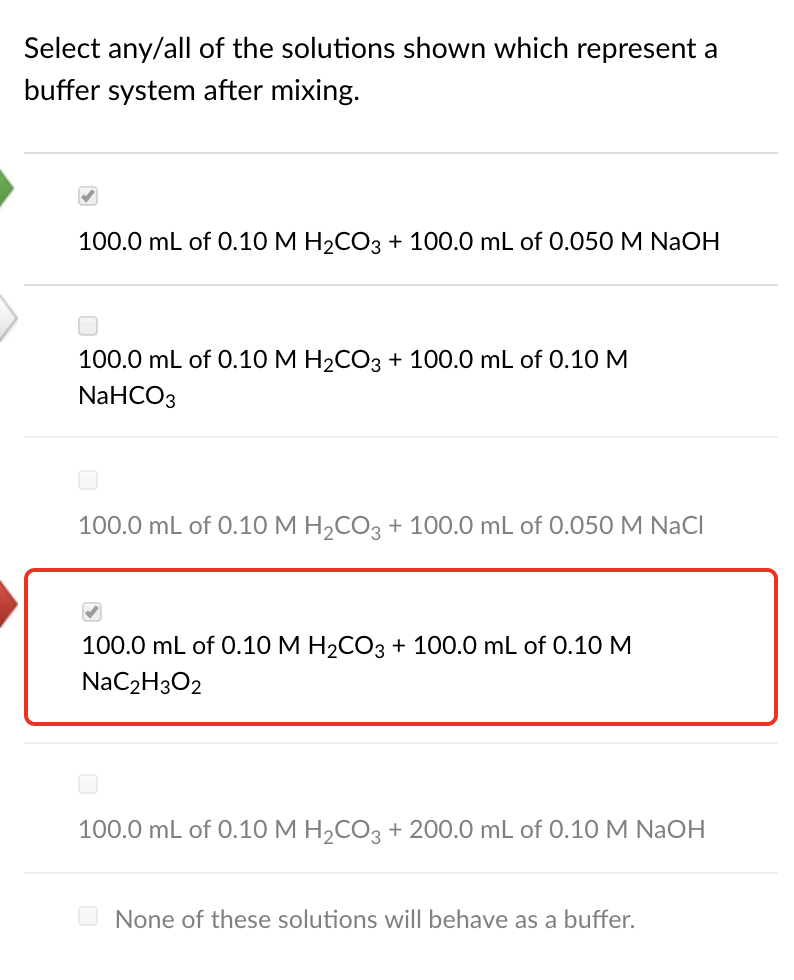
030 M ammonia 037 M ammonium nitrate. Correct answer to the question Which of the following solutions would make a good buffer system.
It is a good buffer.
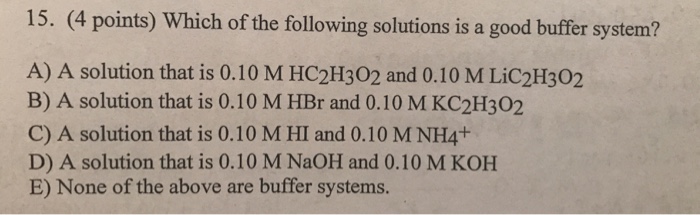
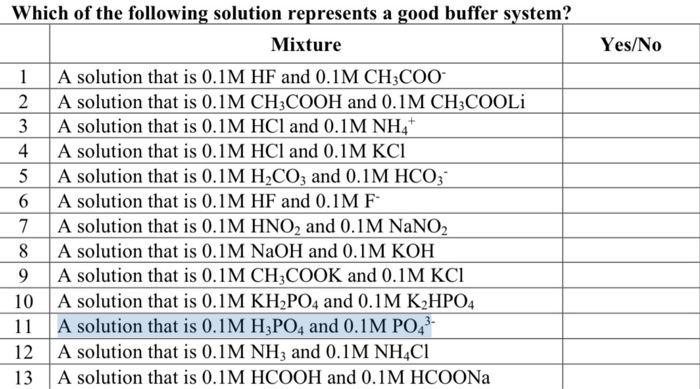
Which of the following solutions is a good buffer system. A a solution that is 010 M HC2H3O2 and 010 M LiC2H3O2 B a solution that is 010 M HF and 010 M NaC2H3O2 C a solution that is 010 M HCl and 010 M NH4 D a solution that is 010 M NaOH and 010 M KOH E None of the above are buffer systems. A solution that is 010 M HCN and 010 M NaF C. A solution that is 010 M HCN and 010 M NaCl A solution that is 010 M HCN and 010 M LiCN A solution that is 010 M HNO3 and 010 M KNO3 A solution that is 010 M NaOH and 010 M HNO3 A solution that is 010 M NaCl and 0. A solution that is 010 M HCN and 010 M KI. Which of the following solutions is a good buffer system. A A solution that is 010 M NaCl and 010 M HCl. Which of the following solutions is a good buffer system. A solution that is 010 m hcn and 010 m nacl a solution that is 010 m hcn and 010 m licn a solution that is 010 m hno3 and 010 m kno3 a solution that is 010 m naoh and 010 m hno3 a solution that is 010 m nacl and 010 m hc.
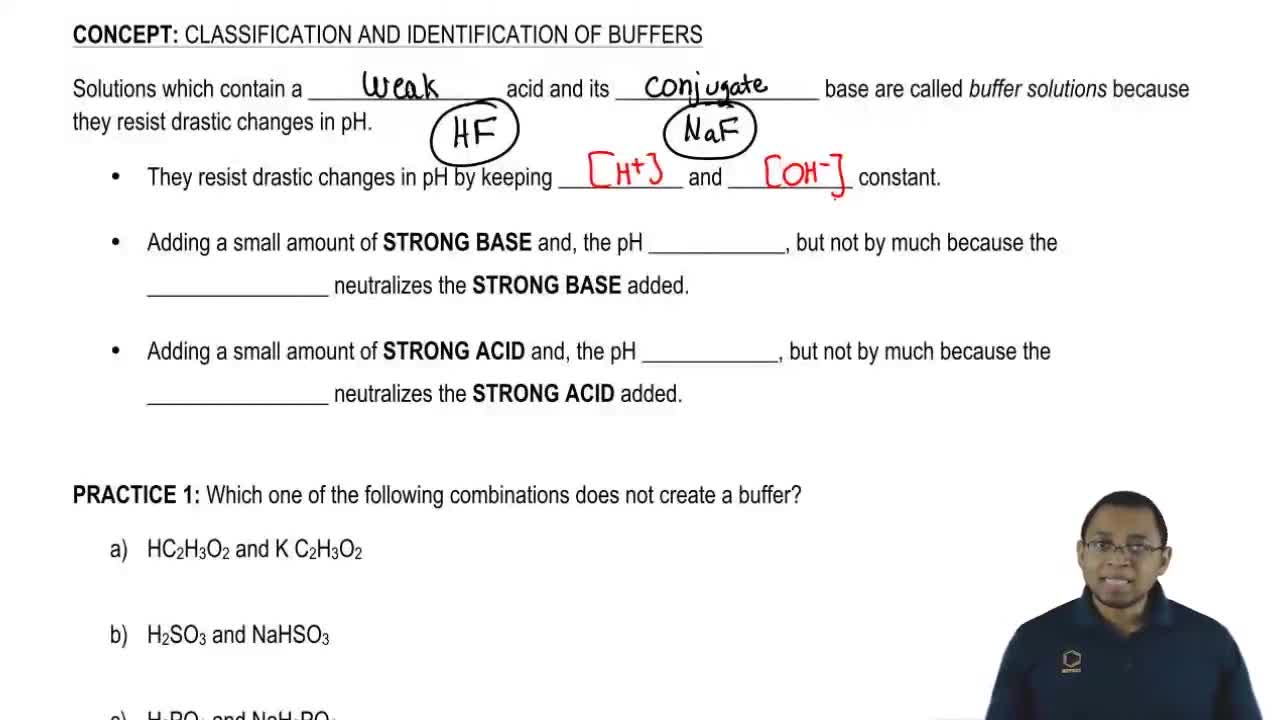
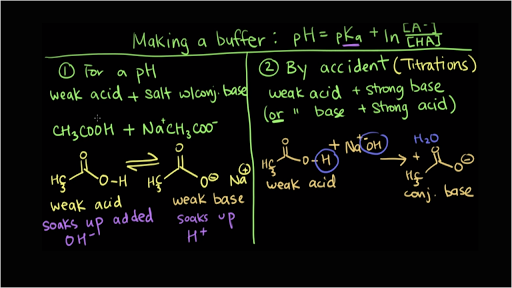
Which of the following aqueous solutions are good buffer systems. A solution that is 010 m hcn and 010 m nacl a solution that is 010 m hcn and 010 m licn a solution that is 010 m hno3 and 010 m kno3 a solution that is 010 m naoh and 010 m hno3 a solution that is 010 m nacl and 010 m hc. A A solution that is 010 M NaCl and 010 M HCl. A buffer is defined as the mixture between a weak acid and its conjugate base or vice versa. A buffer solution contains a weak acid or base and the conjugate base or acid A solution that is 010 M HC2H3O2 and 010 M LiC2H3O2The solution has the same amount of moles in the weak acid and conjugate base. A solution that is 010 m hcn and 010 m nacl a. It is not a buffer system because is not a salt of weak acid HF.
































Post a Comment for "Which Of The Following Solutions Is A Good Buffer System?"